In this episode of MedtronicTalks, Que Dallara, EVP and President of the Diabetes Business at Medtronic, discusses the company's recent advancements in diabetes management.
Dallara, transitioning from the software and technology industry to the medical device industry, draws parallels between control systems in different fields and their application to diabetes management, particularly in the development of closed-loop algorithms for insulin dosing. She also highlights the FDA-approved MiniMed 780G system, which simplifies diabetes management with automatic adjustments for insulin dosing and carbohydrate counting. Finally, Dallara addresses Medtronic's ongoing engagement with the FDA and news of the launch of the MiniMed 780G system in the U.S., sharing more about its meal detection technology and benefits.
Thank you to GlobalMed-MDI for sponsoring this episode. To learn more about how GlobalMed-MDI works with medical device companies, visit www.moldeddevices.com.
Thank you for listening to the Medtronic Podcast.
Subscribe to this podcast on every major podcast platform.
From The Podcast
MedtronicTalks
The MedtronicTalks Podcast Series brings insights, observation and lessons from the leaders of the medtech industry’s single, largest leader. Episodes will center around one of Medtronic’s business units ranging from cardiac care to surgical robotics. We’ll talk with decision-makers who keep Medtronic at the forefront of Medtech. We’ll also speak with regulatory, financial and other operational leaders including the CEO about how Medtronic is preparing for the future.Join Podchaser to...
- Rate podcasts and episodes
- Follow podcasts and creators
- Create podcast and episode lists
- & much more
Episode Tags
Claim and edit this page to your liking.
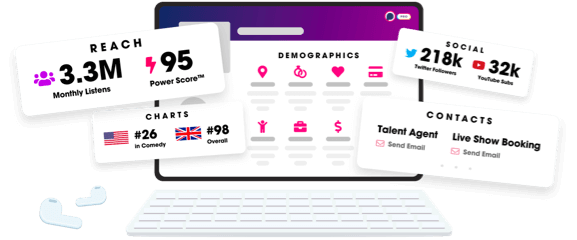
Unlock more with Podchaser Pro
- Audience Insights
- Contact Information
- Demographics
- Charts
- Sponsor History
- and More!
- Account
- Register
- Log In
- Find Friends
- Resources
- Help Center
- Blog
- API
Podchaser is the ultimate destination for podcast data, search, and discovery. Learn More
- © 2024 Podchaser, Inc.
- Privacy Policy
- Terms of Service
- Contact Us
